By: Andrew Moshfeghi, MD, MBA;
This course has expired. You can still review the content but course credit is no longer available.
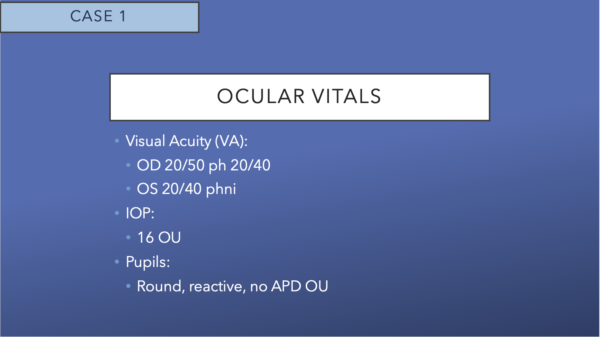
In patients with diabetes, there is often no obvious evidence of diabetic retinopathy (DR), ie, no intraretinal hemorrhages, exudates, or obvious microaneurysms. However, some of the newer imaging modalities can detect minor vascular changes much earlier than previous imaging options. This activity will focus on the importance of screening, overcoming barriers to recommended care, early detection through imaging modalities, and the importance of timely referral to a retina specialist for advanced imaging.
This activity is supported by an independent medical education grant from Carl Zeiss Meditec and Regeneron Pharmaceuticals, Inc.
Upon completion of this activity, the participant should be able to:
Sponsored by:
Evolve is an approved COPE Administrator.
This course is COPE approved for 0.5 hours of CE credit for optometrists.
In order to obtain credit, proceed through the program, complete the post-test, evaluation and submit for credit.
Course Viewing Requirements
Supported Browsers:
Internet Explorer 11 for Windows
Edge (recent versions; Chromium-based) for Windows
Google Chrome (recent versions) for Windows, Mac OS, iOS, Android, or Linux
Mozilla Firefox (recent versions) for Windows, Mac OS, iOS, Android, or Linux
Safari (recent versions) for Mac OSX, or iOS
Hardware Requirements:
4GB+ RAM
Recommended internet speed 5Mbps+

Andrew A. Moshfeghi, MD, MBA (Program Chair)
Associate Professor of Clinical Ophthalmology
Medical Director of the USC Roski Eye Institute
Director of Clinical Trials
Director of the Vitreoretinal Surgery Fellowship Program
Director of the Medical Retina Fellowship Program
Los Angeles, CA
Group Leaders

Dilsher Dhoot, MD
California Retina Consultants/Retina Consultants of America
Santa Barbara, CA

David Eichenbaum, MD
Clinical Assistant Professor
Department of Ophthalmology
University of South Florida College of Medicine
Retina Vitreous Associates of Florida
Tampa, FL

Avni P. Finn, MD
Vitreoretinal Surgeon
Northern California Retina Vitreous Associates
Mountain View, CA

Roger A. Goldberg, MD, MBA
Partner, Bay Area Retina Associates
Faculty, CPMC Ophthalmology Residency
San Francisco Bay Area, CA

Esther Lee Kim, MD
Vitreoretinal Surgeon Orange County Retina
Santa Ana, CA

Lisa C. Olmos de Koo, MD, MBA
Associate Professor of Ophthalmology
Subspecialty Chief, Retina Division
Program Director, Vitreoretinal Surgery Fellowship
Department of Ophthalmology
University of Washington
Seattle, WA

Sonia Mehta, MD
Assistant Professor of Ophthalmology
Thomas Jefferson University School of Medicine
Philadelphia, PA

Ehsan Rahimy, MD
Vitreoretinal Disease & Surgery
Palo Alto Medical Foundation
Palo Alto, CA

Adrienne W. Scott, MD
Associate Professor of Ophthalmology
Retina Division
Wilmer Eye Institute
Johns Hopkins University School of Medicine
Baltimore, MD

Jayanth Sridhar, MD
Department of Ophthalmology
Bascom Palmer Eye Institute
Miami, FL
DISCLOSURE POLICY
It is the policy of Evolve that faculty and other individuals who are in the position to control the content of this activity disclose any real or apparent conflict of interests relating to the topics of this educational activity. Evolve has full policies in place that will identify and resolve all conflicts of interest prior to this educational activity.
The following faculty/staff members have the following financial relationships with commercial interests:
Andrew Moshfeghi, MD, has had a financial agreement or affiliation during the past year with the following commercial interests in the form of Consultant: Allergan, Genentech, Graybug, Novartis, Ocular Therapeutix, Pr3vent and Regeneron. Grant/Research Support: Genentech, Novartis, and Regeneron. Stock/Shareholder: Ocular Therapeutix, Pr3vent, and Placid.
OFF-LABEL STATEMENT
This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. The opinions expressed in the educational activity are those of the faculty. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
DISCLAIMER
The views and opinions expressed in this educational activity are those of the faculty and do not necessarily represent the views of Evolve, Carl Zeiss Meditec or Regeneron Pharmaceuticals, Inc.
In patients with diabetes, there is often no obvious evidence of diabetic retinopathy (DR), ie, no intraretinal hemorrhages, exudates, or obvious microaneurysms. However, some of the newer imaging modalities can detect minor vascular changes much earlier than previous imaging options. This activity will focus on the importance of screening, overcoming barriers to recommended care, early detection through imaging modalities, and the importance of timely referral to a retina specialist for advanced imaging.
On a global scale, many epidemiologic studies have shown that approximately 1 in 3 people with diabetes has diabetic retinopathy (DR), and 1 in 10 has proliferative DR (PDR) or diabetic macular edema (DME). Based on these rates, between 100 million and 120 million people worldwide have DR and possibly 20 million to 30 million have PDR or DME. Further, population surveys consistently show that half of those with diabetes remain undiagnosed and many are unaware of their risk of DR and other complications.
According to the American Academy of Ophthalmology’s Guidelines on Diabetic Eye Care: The International Council of Ophthalmology Recommendations for Screening, Follow-up, Referral, and Treatment Based on Resource Settings published in 2018, vision loss resulting from DR can be prevented with a broad-based systems-level approach. This could begin by increasing public knowledge with targeted health care education, followed by well-implemented screening programs for every person with DM. Next, timely referral for patients with more severe levels of DR, and finally, appropriate treatment for patients with advanced DR, such as PDR and DME.
Although public health programs like those mentioned could be implemented in high-income countries and communities with appropriate funding, trained health care manpower, access to medications, and diagnostic and surgical facilities, these programs remain a substantial challenge in areas without access to these resources.1
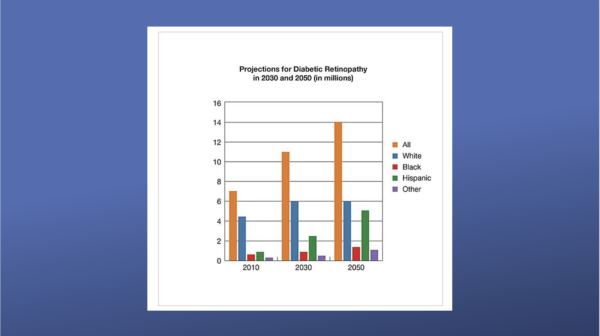
From 2010 to 2050, the number of Americans with DR is expected to nearly double, from 7.7 million to 14.6 million. Hispanic Americans are expected to see the greatest increase in cases, rising more than three-fold from 1.2 million to 5.3 million.2, 3
In the United States, updated estimates indicate there are approximately 84 million Americans with prediabetes and more than 34 million with diabetes. Only 26.9 million are diagnosed with diabetes, leaving 7.3 million undiagnosed, according to the Centers for Disease Control and Prevention (CDC).
About 7.7 million of those diabetics have DR but only 2 million are diagnosed and only 1 million are being treated, according to 2010 estimates (latest available figures).
However, among US adults aged 18 years or older with diagnosed diabetes, crude data for 2018 indicated that 11.7% (95% CI, 11.0%–12.5%) reported vision disability, including blindness.4-7

As mentioned, appropriate screening programs could save vision in patients with DR. However, many of those who have access to screening and treatment do not seek it.
According to the National Eye Institute (NEI), some patients with DR may need a comprehensive dilated eye exam as often as every 2 to 4 months, yet only about 63% of adults with diabetes have received only one comprehensive eye exam during the past year.1, 7-9

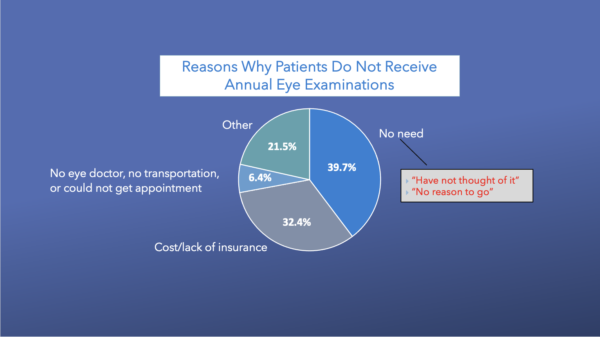
Among adults with diagnosed diabetes, nonadherence to the recommended annual eye examinations was 23.5%, according to 2006–2010 Behavioral Risk Factor Surveillance System data from 22 states (n = 27,699).
The most commonly reported reasons for not receiving eye care in the preceding 12 months were “no need” and “cost or lack of insurance” (39.7 and 32.3%, respectively). Other reasons were “no eye doctor,” “no transportation” or “could not get appointment” (6.4%), and “other” (21.5%). After controlling for covariates, adults aged 40–64 years were more likely than those aged 65 years and older (relative risk ratio [RRR] = 2.79; 95% CI 2.01–3.89) and women were more likely than men (RRR = 2.33; 95% CI 1.75–3.14) to report “cost or lack of insurance” as their main reason. However, people aged 40–64 years were less likely than those aged 65 and older to report “no need” (RRR = 0.51; 95% CI 0.39–0.67) as their main reason.10
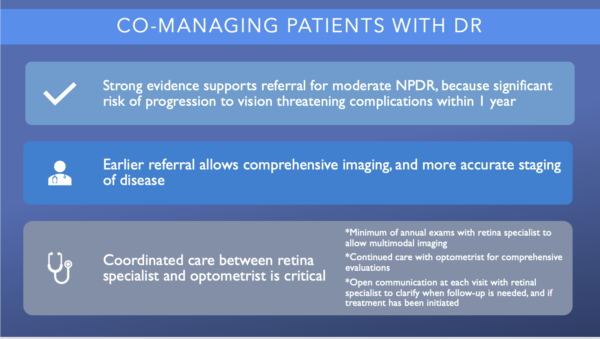
Strong evidence supports referral to a retina specialist for patients with moderate nonproliferative diabetic retinopathy (NPDR) because significant risk of progression to vision threatening complications within 1 year. Earlier referral allows comprehensive imaging, and more accurate staging of disease.
Coordinated care and communication between the optometrist and retina specialist is critical. The optometrist should continue to provide comprehensive evaluations, but the patient should see a retina specialist at least annually to allow for multimodal imaging and to establish a relationship. It is reasonable for the optometrist and retina specialist to communicate after each visit to clarify when follow-up is needed, and to discuss if treatment was initiated.1, 11



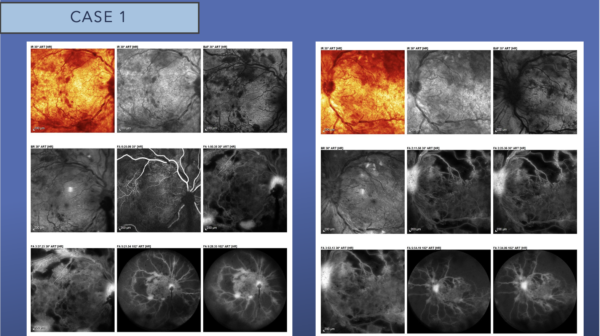
Fluorescein angiography (FA) reveals blockage secondary to extensive retinal hemorrhages scattered throughout the posterior pole. There is early and late leakage at the disc in both eyes, as well as along the inferotemporal arcade in the right eye. There is extensive capillary nonperfusion seen immediately outside the macula, into the periphery in both eyes. This is a picture of very high-risk PDR.

OCT confirms the presence of diffuse intraretinal fluid and cystoid macular edema. There is a small amount of subretinal fluid at the fovea in both eyes. A few tufts of NVD can be seen at the disc, as well.

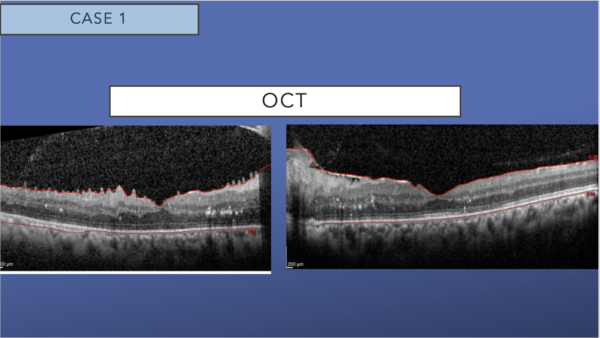
OCT shows dramatic resolution of the intraretinal and subretinal fluid, with residual hard exudates in both eyes. A mild epiretinal membrane is present in both eyes.


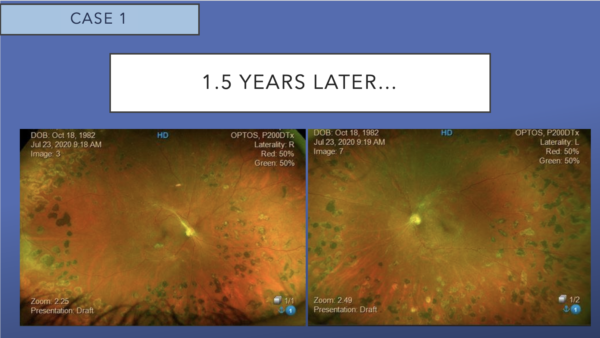
Color fundus photos show 360-degree panretinal laser photocoagulation, regressed NVD and NVE. There is significant reduction in the size and quantity of intraretinal hemorrhages OU.
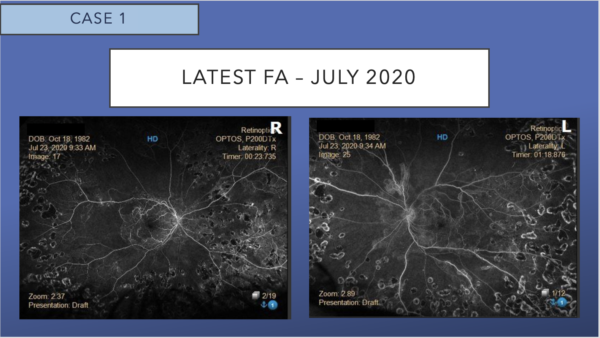
Corresponding FA shows the absence of leakage and persistent peripheral nonperfusion.
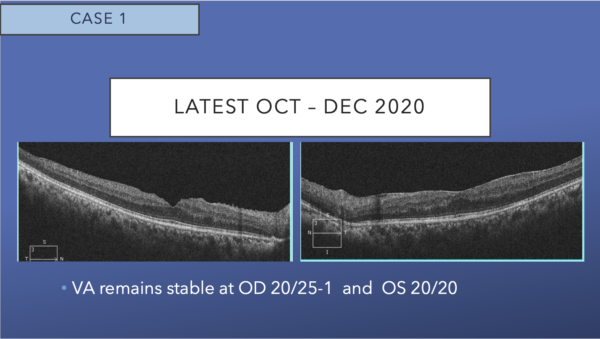
OCT shows mild epiretinal membrane, and resolution of cystoid macular edema, subretinal fluid, and hard exudates in both eyes. The excellent appearance of his OCT corresponds to his excellent VA.
Decreasing barriers to patient care can begin by better utilizing electronic medical records (EMR) to communicate with the patient’s other health professionals. Efforts to prevent DR development are generally focused on diabetes and hypertension management because poor glycemic control and elevated blood pressure have consistently been shown to exacerbate DR development and progression. Early detection and management of DR are also widely recognized as being key to reducing diabetes-associated vision loss.
Educational programs for patients can also be helpful. Awareness of the need for detecting DR at a symptomless stage is a key factor in uptake and regular follow up.
Finally, reducing vision impairment from DR requires greater emphasis on timely adherence to ophthalmology referral and follow-up. Prevention of visual loss with retinopathy screening, but must include patient engagement, adherence monitoring, and streamlining ophthalmic referral and management. This can be streamlined when optometric and ophthalmic practitioners are in a single practice.1, 12-14
In recent years, there have been five major technological advances in imaging leading to improved DR detection.15-17
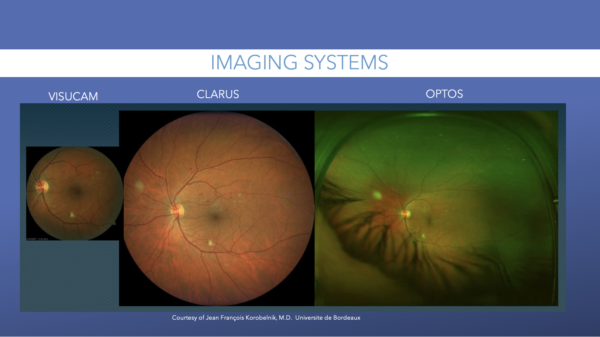
Fundus photography is best used for quick image acquisition, and it is generally comfortable for patients. There are two types of fundus images: standard and ultra widefield (UWF). For many years, the standard method for screening and grading severity of diabetic retinal disease relied upon a mixture of photographs using normal-angle fundus cameras.
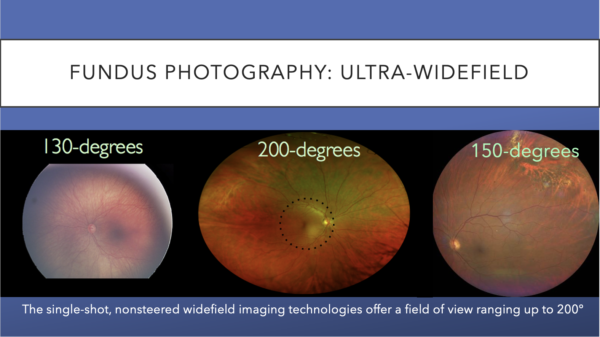
Widefield images can be montaged to create an UWF field of view, but that technique requires significantly more time for capture and montaging.18-19

The single-shot, nonsteered widefield imaging technologies offer a field of view ranging up to 200-degrees. These images offer examples of views ranging from 130-degrees to 200-degrees.
The development of ultra-widefield (UWF) retinal imaging has accelerated our understanding of common retinal diseases. The imaging allows more of the retina to be captured with fewer pictures, which translates to less dependence on photographer skill. The process is also more comfortable for the patient. Recent studies have shown comparability between traditional and UWF imaging for standard grading of DR. Further, UWF images can detect peripheral pathology not typically seen in standard photographs.
Previously, UWF and widefield imaging were variably defined based on degrees of freedom of the retinal capture using device-specific terminology. DRCR.net previously defined UWF images to have at least 100° view of the fundus. Recently, a consensus group of retinal imaging experts led by Netan Choudhry, MD, formalized a definition of the terms based on anatomic landmarks. Based on their consensus, widefield images were defined as a single-capture image, centered on the fovea, which captures retinal anatomic features beyond the posterior pole, but posterior to the vortex vein ampulla, in all four quadrants. UWF was defined as a single-capture image, centered on the fovea, which captures retinal anatomic features anterior to the vortex vein ampullae in all four quadrants.
Ischemia, an important factor in DR progression, may appear in the periphery first and has been associated with the presence of peripheral lesions on color images. Compared to the seven-standard field (SSF) images, UWF-FA has higher utility in demonstrating peripheral pathology, imaging 3.9 times more nonperfusion, 1.9 times more neovascularization, and prompting 3.8 times more panretinal photocoagulation in one study. A review article by Michael A. Klufas, MD, and colleagues also showed that UWF-FA detected findings that would have otherwise been missed on SSF imaging in 10% of eyes. Correlations between peripheral nonperfusion/vascular leakage and neovascularization, peripheral ischemia and an enlarged foveal avascular zone, and peripheral nonperfusion and diabetic macular edema show that the ability to detect abnormalities in the periphery may have clinically significant implications.18-25
Telemedicine-based DR screening incorporating artificial intelligence technology has the potential to facilitate more widespread and cost-effective screening, particularly in low- and middle-income countries.
The utility of telemedicine has grown during the COVID-19 pandemic, and telemedicine applications for UWF imaging have been established and are likely to expand, particularly with the increasing prevalence of diabetes worldwide, according to Klufas and colleagues. For example, within the endocrinology department of a multispecialty private hospital, UWF fundus photography resulted in the detection of DR in 9.3% of 1,024 screened patients.
Recently, a novel hybrid telemedicine system using both fixed (located within high-volume clinics) and mobile (mounted within a traveling van) UWF cameras allowed for the screening of 2,788 diabetic patients and detection of DR in 27% of patients.
Imaging is also being incorporated into artificial intelligence (AI) medical devices. An FDA-approved, autonomous, artificial intelligence (AI)-based diagnostic system is used with fundus imaging to detect the presence of DR. Although this system uses widefield imaging (four pairs of digital images with visualization of 45°–60° by each pair), there may be a potential role for use with UWF imaging in the future. 26-28, 40

In a prospective study of 819 patients, the AI-based diagnostic system met predetermined sensitivity and specificity endpoints for the autonomous detection of more than mild DR, inclusive of DME, in people with diabetes in primary care settings.
The AI system was evaluated relative to standardized imaging and grading protocols by the Wisconsin Fundus Photograph Reading Center (FPRC). The grading included Early Treatment Diabetic Retinopathy Study Severity Scale (ETDRS) and DME determinations from widefield stereoscopic photographs and macular OCT. More than mild DR was defined as ETDRS level 35 or higher, and/or DME, in at least one eye.
Of the 900 participants were prospectively enrolled, a subset of 819 participants could be fully evaluated by both AI and FPRC, and 198 (23.8%) had more than mild DR; the AI system detected more than mild DR at a sensitivity of 87.2% (95% CI, 81.8-91.2%) and specificity of 90.7% (95% CI, 88.3-92.7%). Image- ability, the percentage of participants with completed FPRC grading and a disease level AI output, was 95.6% (95.0% CI, 94.0-96.8%).
The study was sponsored by IDx.29

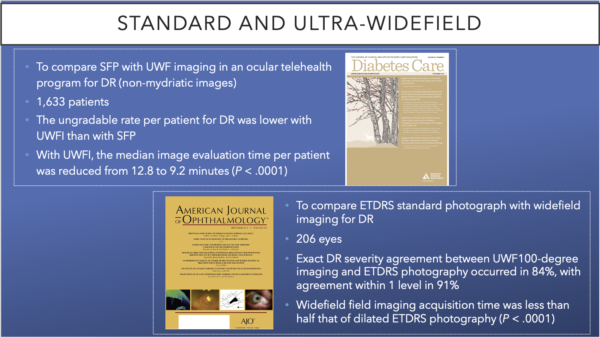
Wide angle fundus imaging gets past small pupils and media opacities. Various clinical studies have compared standard fundus photography (SFP) and UWF. Two studies by Silva et al, published in the AAO’s American Journal of Ophthalmology and the ADA’s Diabetes Care, concluded that UWF reduced evaluation time for patients.
The AAO journal’s retrospective study compared ETDRS standard photography with UWF for DR in 206 eyes. Both 200-degree UWF images and UWF FA images were acquired at the same visit. ETDRS templates were overlaid digitally based on disc and macula location onto stereographically projected UWF images. Exact DR severity agreement between UWF 100-degree imaging and ETDRS photography occurred in 84%, with agreement within 1 level in 91%. UWF acquisition time was less than half that of dilated ETDRS photography (P < .0001).
The authors concluded that the evaluation of UWF FA for nonperfusion area and NPI is reproducible, following a standardized protocol. “Both parameters were correlated highly with the presence of predominantly peripheral lesions (PPLs) and DR severity. Given that the presence and extent of PPLs have been associated with increased risks of DR progression, the clinical identification of PPLs may reflect closely the extent of nonperfusion and ischemia, thus accounting for the increased risk of progression,” the authors wrote.
The ADA journal study, which included 1,633 patients, reported that evaluation time per patient was reduced from 12.8 to 9.2 minutes (P < .0001) and the authors concluded that UWF imaging demonstrated good sensitivity and specificity for detection of DR and for identification of referable retinal disease by nonphysician imagers when used in telemedicine.
However, the ungradable rate per patient for DR was lower with UWF imaging than with SFPs. The authors further concluded, “With immediate image evaluation, less than 0.1% of patients with referable DR would be missed, reading center image grading burden would be reduced by 60%, and patient feedback would be expedited.”18, 23, 30
Fourier-domain OCT (FD-OCT) can be characterized into two types: spectral-domain OCT (SD-OCT) and swept source OCT (SS-OCT).
With the development of time-domain OCT (TD-OCT) and the more recent FD-OCT, high quality images of the retinal structure are now clearly demonstrated, such as inner limiting membrane (ILM), retinal nerve fiber layer (RNFL), photoreceptor inner and outer segments (IS/OS), and outer limiting membrane. Even though SD-OCT and SS-OCT both use the Fourier transform, SD-OCT instruments use a broadband near infrared superluminescent diode as a light source with a spectrometer as the detector.
SD-OCT has greatly improved speed and sensitivity and is able to detect small changes in morphology of retinal layers and choroidal neovascularization (CNV) activity. However, SD-OCT has difficulty differentiating vascular and fibrous components of CNV because of their similar reflectivity properties.15

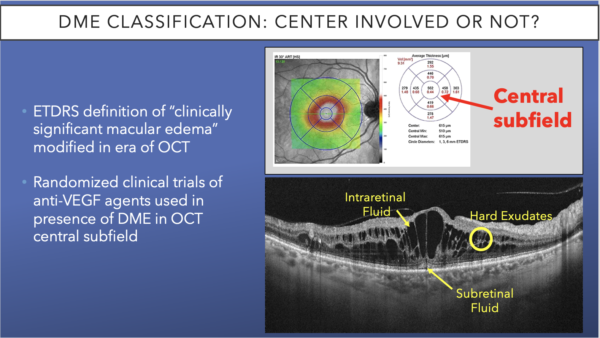
When examining a patient, one of the main parameters for classifying DME is whether it is center involved, ie, involving the area of the fovea, including the down slope of the fovea contour, as seen in the top image. If there is center-involved DME (CI-DME), next you must consider whether there is vision loss.
On the OCT, look for pockets of intraretinal fluid and subretinal fluid, as well as hard exudates. These are all classic signs of DME, for which the patient should be treated.31-32

This 30-degree color fundus photo of the left eye shows juxtafoveal lipid, with scattered microaneurysms through the macula, with nonfoveal late leakage within the temporal and inferior macula. The foveal avascular zone is slightly enlarged.
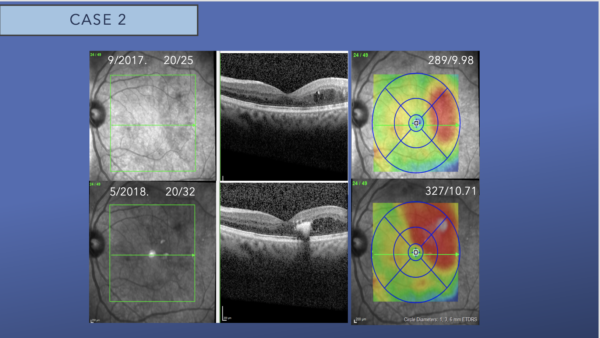
The top row shows SD-OCT of the left eye with DME involving the temporal subfield. The bottom row shows the SD-OCT of the left eye 8 months later. The DME now involves the foveal center, and there is hyperreflectivity in the foveal consistent with foveal lipid. The central subfield thickness has increased from 289 microns to 327 microns.

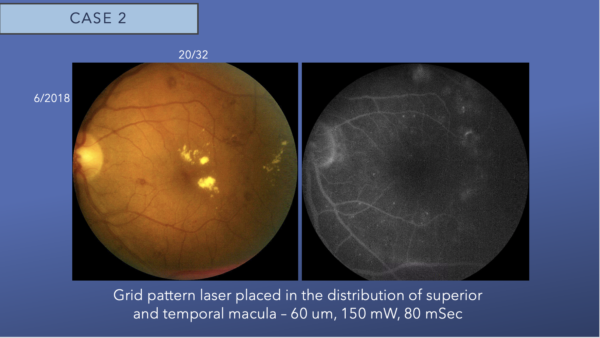
The left eye fundus image shows juxtafoveal lipid and moderate intraretinal hemorrhages. Minimal leakage is noted in the temporal macula on fluorescein angiogram. We placed grid laser in the temporal macula.

Four months following focal laser, intraretinal fluid increased on exam and OCT, and a small new pocket of subfoveal subretinal fluid is observed. Intraretinal hyperreflectivity from consolidated lipid is noted at the fovea.
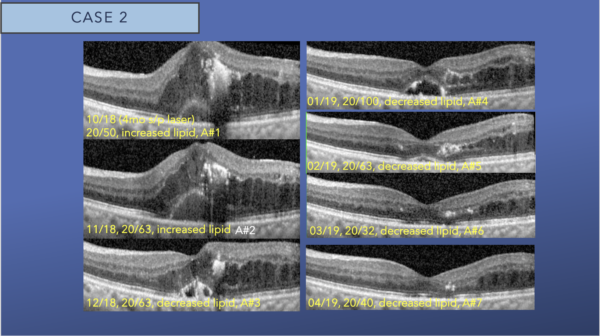
After the third intravitreal aflibercept injection, we see gradual resolution of the foveal lipid and subfoveal subretinal fluid and decreasing intraretinal fluid.
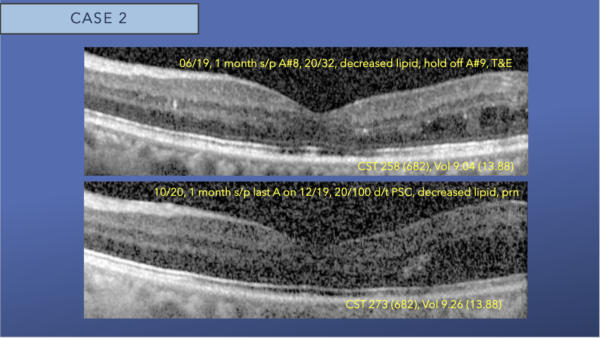
After nine monthly intravitreal aflibercept injections, there is resolution of foveal DME and foveal lipid, with mild interstitial fluid remaining in the temporal macula. We switched to a treat and extend protocol.
The second row OCT, is 2 years after initiation of aflibercept and no central DME is present. The image quality has gradually degraded due to progression of posterior subcapsular cataract.


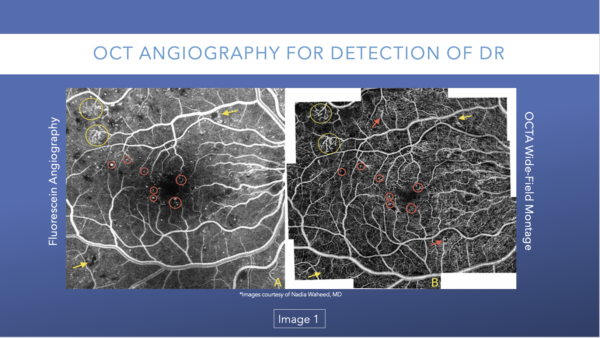
OCT angiography (OCTA) tracks red blood cells as they pass through the vessels. The device captures 11 to 12 images in a row and subtracts them from each other. If anatomy is present, it subtracts that because anatomy doesn’t move. When the reflectance from the blood passing through the vessels, these images are captured and will show plexus of capillaries from these changes. OCTA images are essentially motion-contrast images.
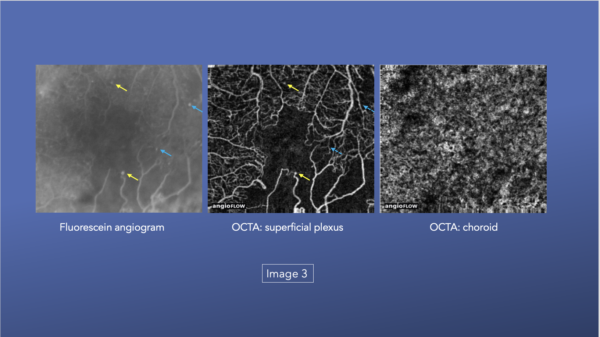
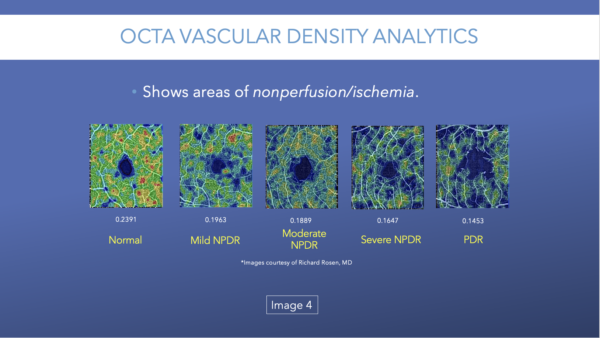
OCTA can detect typical diabetic changes before they are visible clinically, including microaneurysms (Image 1), vascular remodeling (Image 2), and nonperfusion/ischemia (Image 3).
It is easier to detect microaneurysms with OCTA, which translates to earlier/more accurate diagnosis.
Figure 1 compares the same view as shown in FA versus OCTA. While the same microaneurysms and areas of neovascularization are present in both images, the OCTA allows must better visualization.33-35
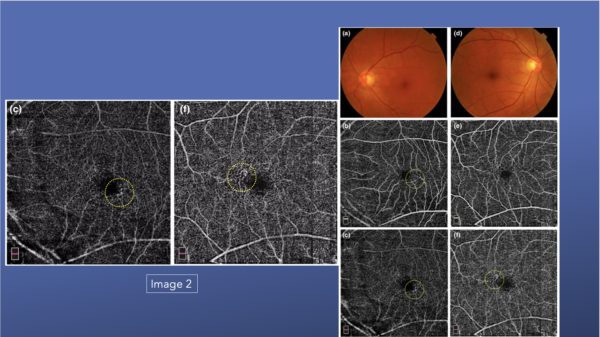
Many patients with DR have vascular remodeling as a result of hyperglycemia. In the fundus photos shown in Image 2, they look relatively clear, but the OCTA images show superficial changes in both the deep plexuses. If looking at the fundus images only, a clinician may suggest seeing the patient again at 12 months or 18 months, but the OCTA images reveal greater changes, even moderate retinopathy, that should be monitored more closely, perhaps seeing the patient again in 6 months.33
Patients can lose vision caused by nonperfusion and/or ischemia and not just edema.33, 36
This series of images also highlight the various levels of DR and how the capillary beds change.33
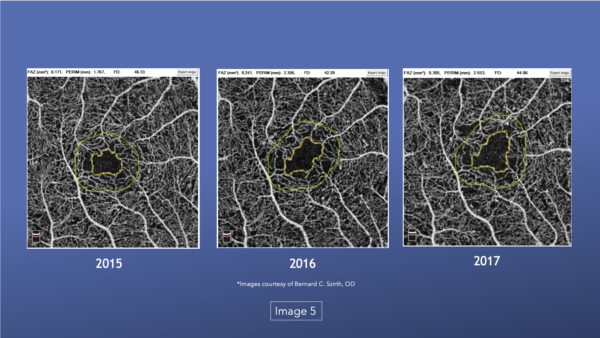
The images also show how the capillary beds changed in a patient over 2 years.33
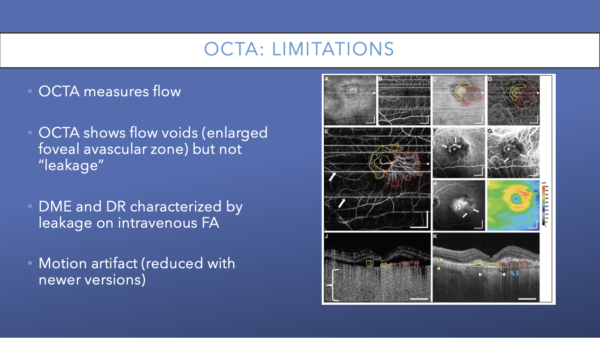
Although OCTA can detect typical diabetic changes before they are visible clinically, there are some limitations to this technology that are worth noting.
OCTA reveals vasculature but a 2018 study by Li and colleagues found that OCTA can have difficulty visualizing microaneurysms and leakage, the two primary reasons to acquire FAs. OCTA represents great improvements in vascular imaging in recent years, but there remains limited ability to evaluate ischemia and hypoxia in tissue microenvironments.35, 40, 15
To develop a consensus on imaging modalities, an international panel, known as the International Widefield Imaging Study Group, with expertise in retinal imaging was assembled to define consensus terminology for widefield imaging and associated terminology. Before the consensus meeting, a set of seven images acquired with a range of imaging methods and representing both healthy and diseased eyes was circulated to the expert panel for independent assignment of nomenclature for each example. The outputs were assembled and used as the starting point for discussions occurring at a subsequent roundtable meeting. The anatomic location, field of view, and perspective provided by each image example was reviewed. A process of open discussion and negotiation was undertaken until unanimous terminology for widefield imaging was achieved.
Across a range of different imaging methods, the expert panel identified a lack of uniform terminology being used in recent literature to describe widefield images. The panel recommended the term widefield be limited to images depicting retinal anatomic features beyond the posterior pole, but posterior to the vortex vein ampulla, in all 4 quadrants. The term ultra widefield was recommended to describe images showing retinal anatomic features anterior to the vortex vein ampullae in all four quadrants. The definitions were recommended over other device-specific terminology.
Current interest in ultra-widefield imaging includes the development of potential biomarkers of disease progression and indicators of preclinical disease development.40, 20

Recent research has begun to highlight the role of peripheral pathology in early disease detection and determination of risk of DR progression. Although the traditional ETDRS SSF includes the central posterior 90° of the retina, which equates to about 30% of the entire retina surface, DR studies have shown that pathology often exists outside the ETDRS SSF, and in some cases, peripheral pathology is associated with greater disease severity and higher risk of disease progression.
Ischemia, which is an important factor in DR progression, may appear in the periphery first and has been associated with the presence of peripheral lesions on color images.40
The ongoing prospective DRCR Protocol AA will further assess whether evaluation of the retinal periphery using UWF imaging, compared to SSF, improves the ability to assess DR and predict worsening disease.
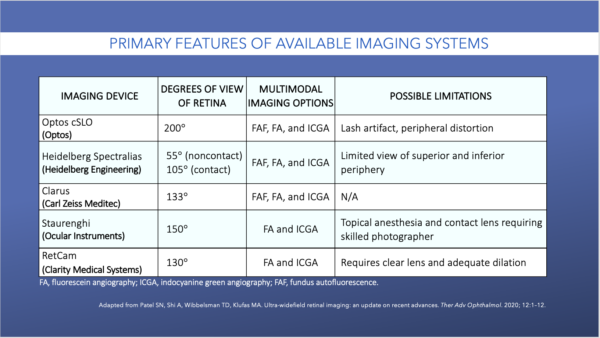
The Table shows the imaging devices that are commercially available.40


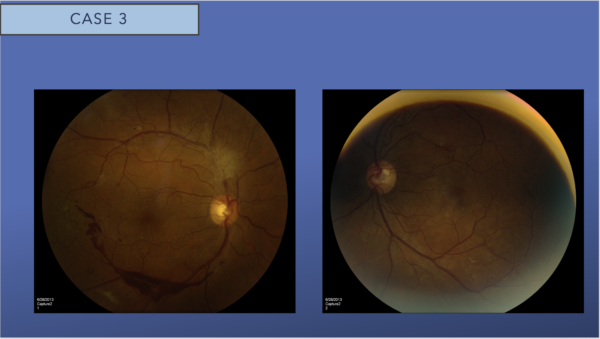
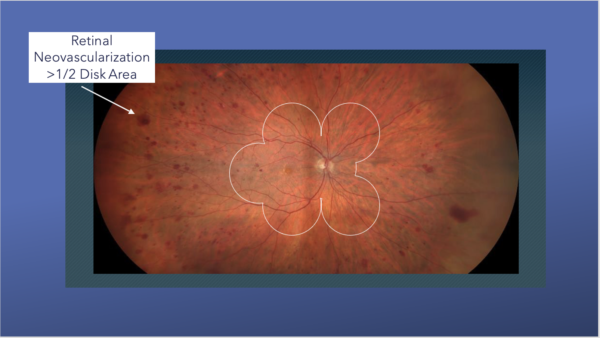
Right eye fundus examination discloses neovascularization of the disc and elsewhere with fibrovascular proliferation superior to the optic nerve and subhyaloid hemorrhage visible along the inferotemporal arcade
Left eye fundus examination reveals evidence of severe nonproliferative diabetic retinopathy with microaneurysms dot blot hemorrhages in all four quadrants but no obvious neovascularization
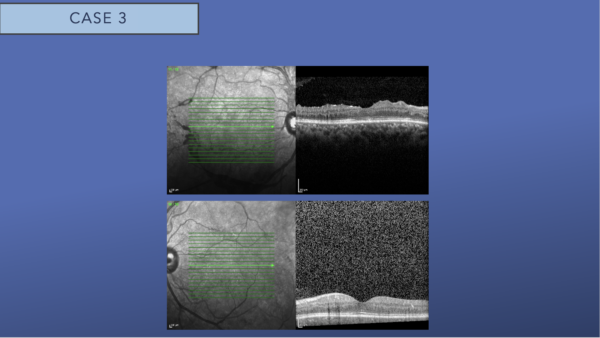
OCT of both eyes revealed no macular edema in either eye.

Fluorescein angiography (FA) late frames reveal nonperfusion and late leakage in the right eye consistent with a diagnosis of high risk proliferative diabetic retinopathy with neovascularization of the disc and elsewhere. FA late frames of the left eye reveal nonperfusion and less prominent leakage consistent with neovascularization elsewhere and a diagnosis of proliferative diabetic retinopathy.
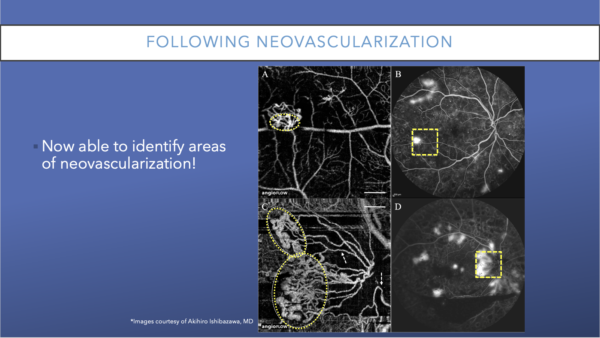
OCTA: Following Neovascularization
OCTA can be used to follow nonperfusion and to also follow neovascularization. This image shows an example of large fronds of neovascularization. Although they were visible on FA, they were much easier to view on OCTA.
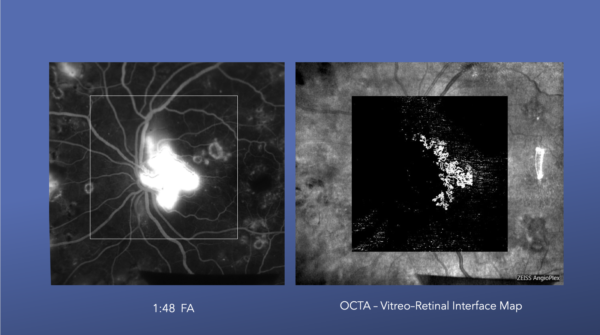
These images illustrate neovascularization of the disc and how it appears on both an FA and an OCTA.
Historically, the standard seven ETDRS fundus photos were used to classify DR severity and they are highlighted in white. Today’s UWF imaging reveals significant additional areas of retina that are easily visible well beyond these ETDRS fundus images. In fact, studies have shown that if a patient has “predominantly peripheral” retinopathy (ie, DR findings outside the standard fields), they are more likely to have a worse long-term prognosis. New imaging techniques, therefore, are an improvement of the prior gold standard in this respect.37, 39
The next big thing in retinal imaging is ultra-widefield swept-source OCTA. There are now machines that can perform OCTA of the entire posterior pole. If all clinics could have these machines and capture these images, there would be no need for FA. Patients would likely prefer it because there would be no dye injections.
These images show advanced diabetic disease that has been progressing for many months to years.38

Technological advances in retinal imaging has allowed for earlier detection of DR. Improvements in fundus imaging during the past two decades has included the development of UWF fundus photography, OCT determination of macular edema, and OCTA assessment of early DR. Peripheral retinal OCT/OCTA are still in development but have promising utility once available. Artificial intelligence and telemedicine may change the way DR screening is performed.
